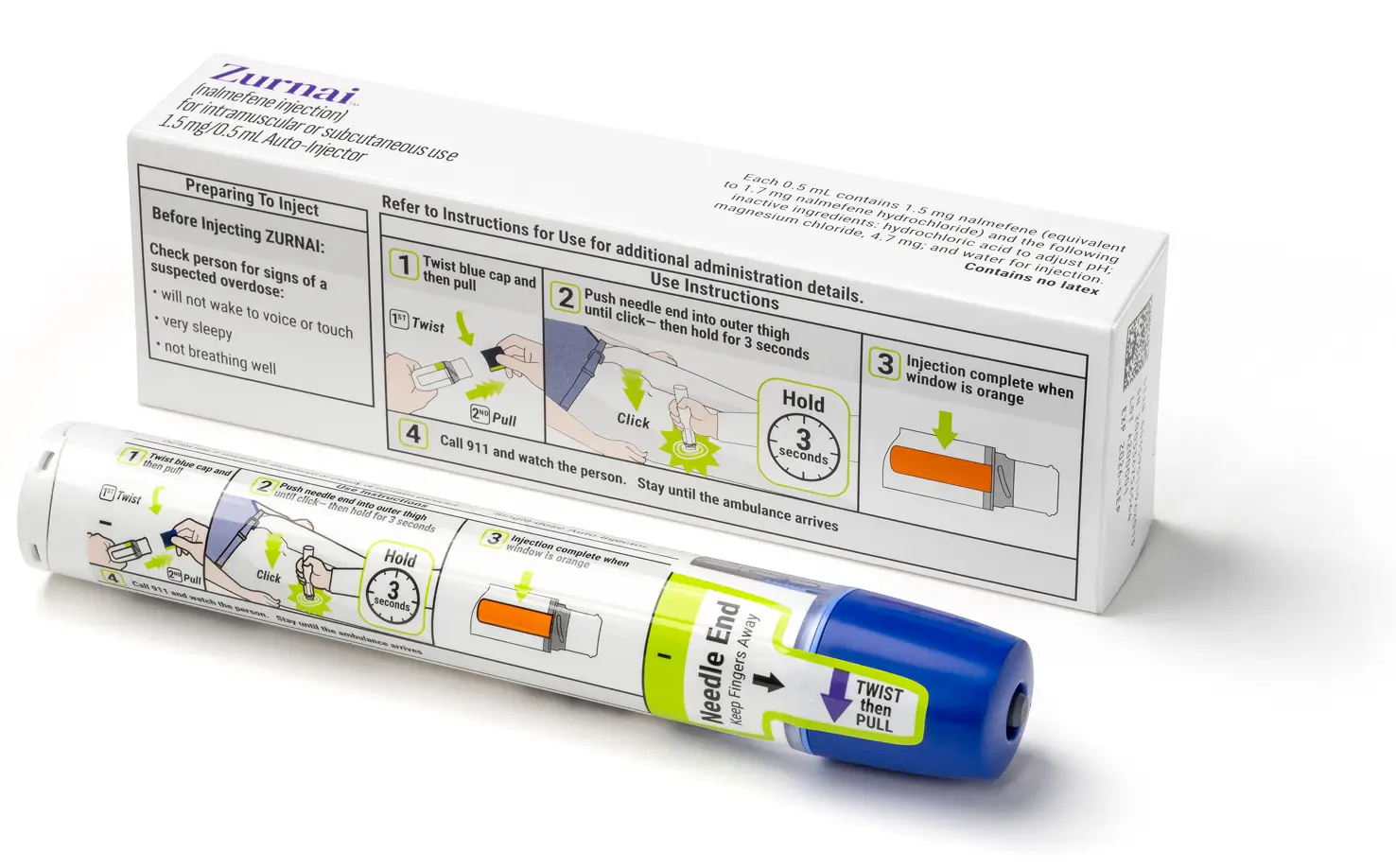
ZURNAI delivers 1.5 mg nalmefene base/0.5 mL in a prefilled, single-dose auto-injector.1
designed to be used by anyone in the community
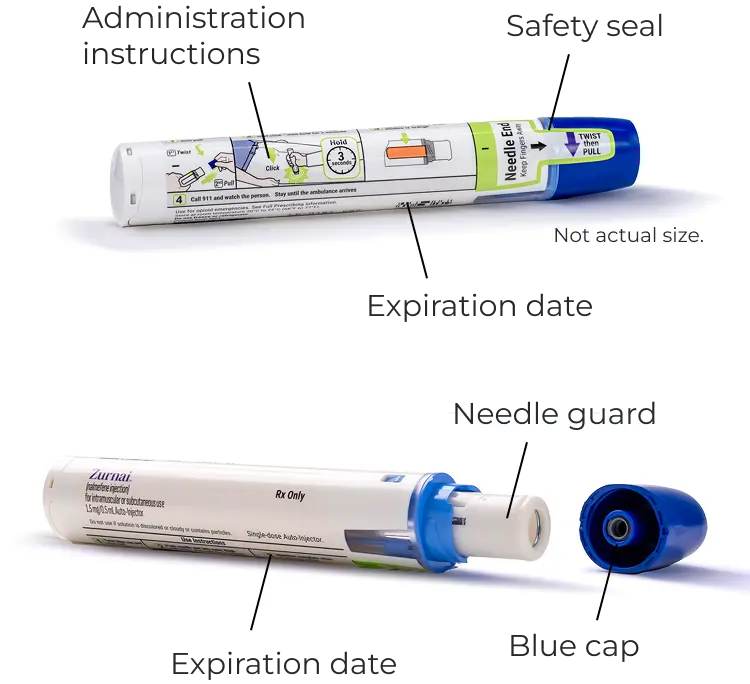
Step-by-step instructions for administering ZURNAI are included on the body of the auto-injector.
Check the expiration date. Replace ZURNAI before the expiration date.
This device includes a safety seal that will break when the cap is removed.
Do not use the device if the safety seal is already broken or missing before use.
Liquid should be clear, colorless to light-yellow and free of particles. You may see air bubbles. This is normal.
Window turns orange when injection is complete.
Reduces chance of accidental needle stick.
Once the safety seal is broken and blue cap is removed, ZURNAI must be used immediately or disposed of properly.
Do not use if safety cap is missing or seal is broken.
 Actor portrayals.
Actor portrayals.how to use ZURNAI2
Refer to for additional administration details.
- ZURNAI is designed to be used by anyone in the community—from first responders to family members.
- Instruct patients and their family members or caregivers to become familiar with all information contained in the carton as soon as they receive ZURNAI.
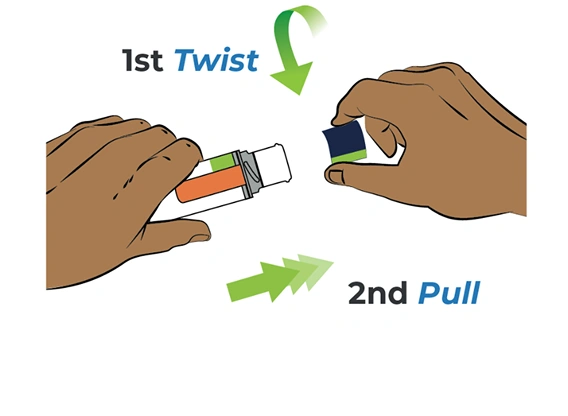
Twist blue cap and
then pull
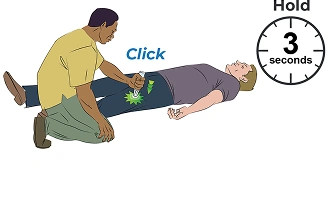
Push needle end into outer thigh until click— then hold for 3 seconds
Injection complete when window is orange
Call 911 and watch the person. Stay until the ambulance arrives
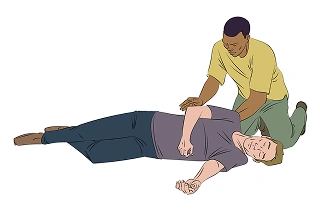
If the person does not wake up within 2–5 minutes after injection, repeat steps 1–3 with a new ZURNAI Auto-Injector.1
Always seek emergency medical assistance after administration of the first dose of ZURNAI in the event of a suspected, potentially life-threatening opioid emergency.1
DID YOU KNOW?
ZURNAI can be administered
through clothing.1