See important information
about administering ZURNAI.
A LONG-ACTING OPTION FOR OPIOID OVERDOSE REVERSAL1*
The first and only nalmefene auto-injector1
While the duration of action of nalmefene is as long as most opioids, a recurrence of respiratory depression is possible, even after an apparently adequate initial response to ZURNAI treatment.
Therefore, it is necessary to seek emergency medical assistance immediately after administration of the first dose of ZURNAI and to keep the patient under continued surveillance. A second dose may be necessary if there is recurrence of symptoms of opioid overdose.
Additional supportive and/or resuscitative measures may be helpful while awaiting emergency medical assistance.
*ZURNAI mean elimination half-life of 9.07 hours is based on a pharmacokinetic study of 24 healthy adult subjects given a single dose of intramuscular ZURNAI.
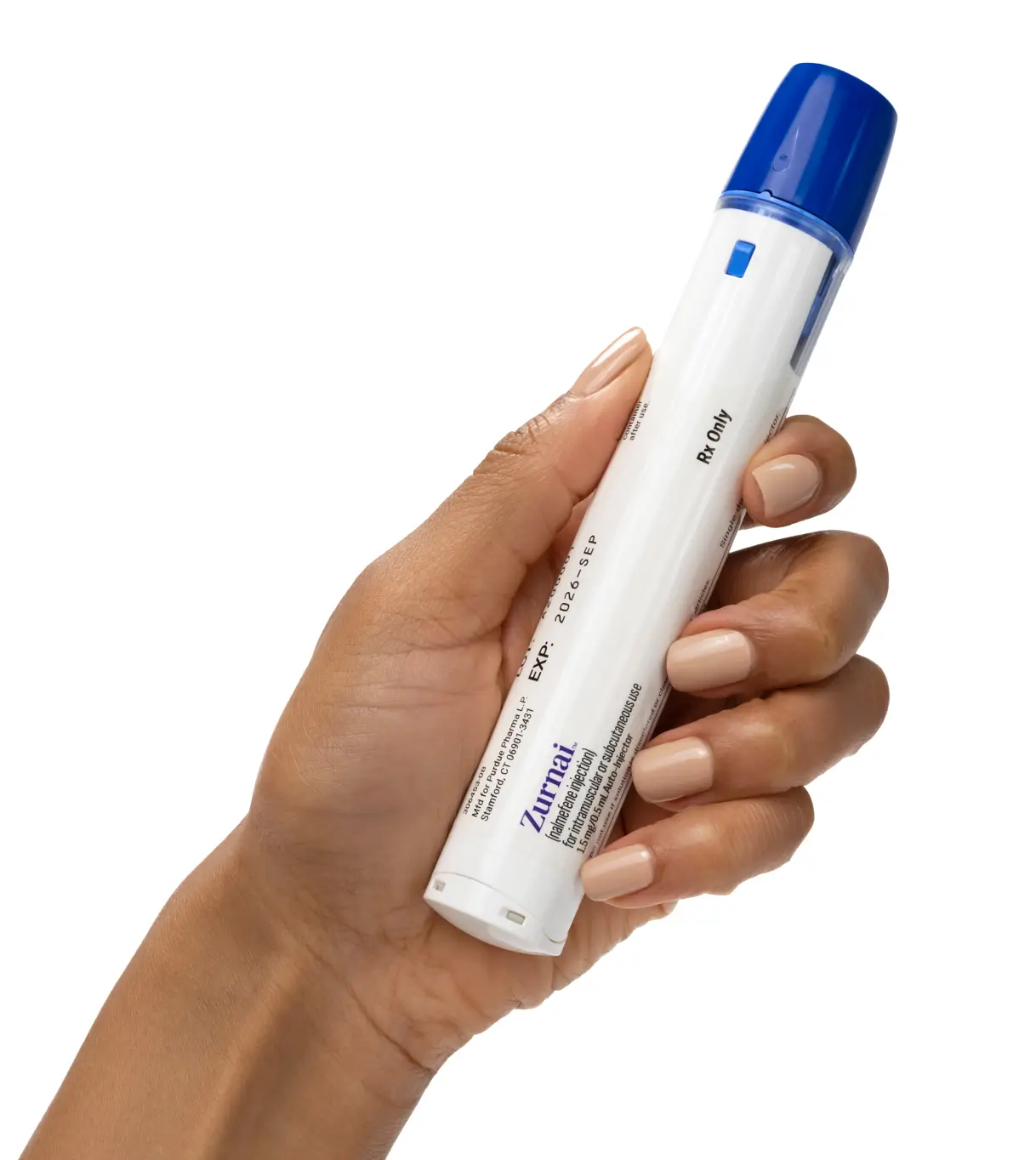
Not actual size.
>80% of opioid receptors blocked within 5 minutes1
>80% of opioid receptors blocked within 5 minutes1
Following a 1 mg parenteral dose, nalmefene was rapidly distributed. In a pharmacology study of brain receptor occupancy, a 1 mg dose of nalmefene blocked more than 80% of brain opioid receptors within 5 minutes after administration.1
ZURNAI (nalmefene injection) is designed to be used by anyone in the community
9-hour mean terminal
elimination half-life1
ZURNAI mean terminal elimination half-life of 9.07 hours is based on a pharmacokinetic study of 24 healthy adult subjects given a single dose of intramuscular ZURNAI.
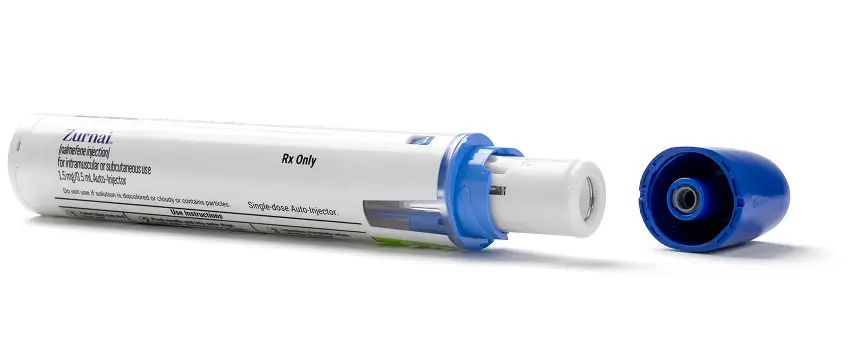
Not actual size.